Acute African sleeping sickness, a devastating parasitic illness occurring primarily in Eastern and Southern Africa, now has a safer, more effective treatment option. Past medical interventions carried up to a 10% fatality rate, but the new oral medication fexinidazole has demonstrated a 97% cure rate in clinical trials. This scientific breakthrough is the result of persistent work by the Drugs for Neglected Diseases initiative (DNDi) in partnership with pharmaceutical company Sanofi and the World Health Organization.
Sleeping sickness is the common name of the parasitic disease human African trypanosomiasis. There are two types of sleeping sickness, often called acute and chronic. Chronic sleeping sickness, the more common type, is caused by T.b. gambiense. Fexinidazole, an inexpensive oral drug made by Sanofi, was approved for this form of the disease in 2018 and replaced previous treatment regimens.
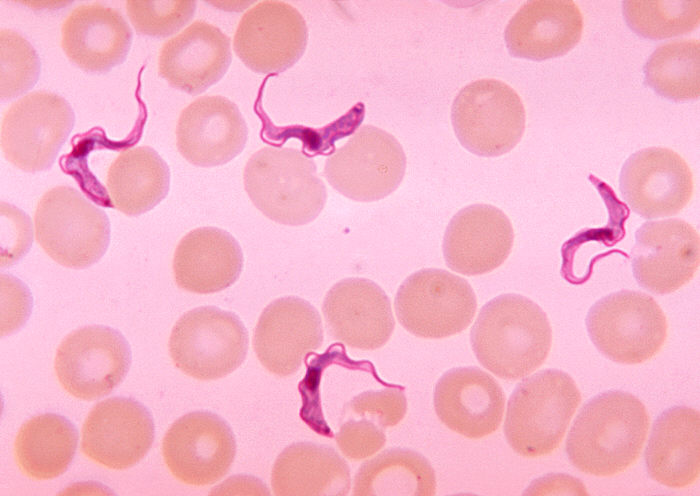
Now, fexinidazole has been approved for the less common acute form of the disease, caused by T.b. rhodesiense. Acute sleeping sickness progresses more rapidly than the chronic form, and often causes death within weeks or months if untreated. Fexinidazole will replace the previous drugs used for treatment: suramin, which required five intravenous injections over a month for stage 1 disease, and melarsoprol, a toxic arsenic-based drug for stage 2 disease.
DNDi, a non-profit organization, has been dedicated to developing treatments for vulnerable communities affected by neglected tropical diseases such as African sleeping sickness. They led the clinical testing of fexinidazole. Approval for the medication offers new hope to impoverished nations that have witnessed thousands succumb during frequent outbreaks. While animal reservoirs mean that complete elimination of the illness is unlikely, experts say the drug’s efficacy and accessibility can mitigate future epidemics.
However, the challenges of diagnosis, distribution to remote areas, and securing continued engagement persist. DNDi stresses that even with this advance, progress could regress without sustained effort. But after years of lacking options beyond toxic and difficult to administer treatments, doctors finally have an orally-administered medication to battle acute sleeping sickness. Thanks to initiatives like DNDi refusing to accept the status quo, the end of this ancient scourge devastating African communities might be within reach.
Related Articles
Borgen Magazine: Alleviating Acute Sleeping Sickness
DNDi: Highly effective and safe all-oral cure for an acute and lethal form of sleeping sickness